Stage-dependent abnormalities induced by the fungicide triadimefon in the mouse.
Francesca Di Renzo, Maria Luisa Broccia, Erminio Giavini, Elena Menegola
Index: Reprod. Toxicol. 31(2) , 194-9, (2011)
Full Text: HTML
Abstract
Aim of this work is the study of abnormalities induced by the triazole triadimefon (FON) administered to pregnant mice at E8, E9, E10, E11 or E12. Pregnant CD-1 mouse were gavaged with FON 500 mg/kg at the selected stages and sacrificed at term and fetuses morphologically examined and processed for visceral and skeletal analysis. Administration of FON on E8, E10-E12 resulted in fetuses with cleft palate (E8 39% and E12 24% representing the peak of sensitivity, in E8 fetuses associated to severe skull basis abnormalities). Other cranial malformations (fusions abnormalities or agenesis of bones) were observed in E8-E10 groups (E8 the most sensitive with 96% of malformed fetuses). Cardiovascular abnormalities were observed in a stage dependent manner at E8-E10 (22.2, 3.8, 7.8%). As far as craniofacial malformation is concerned, we propose that FON acts on two different stages, involved in early and late craniofacial formation.Copyright © 2010 Elsevier Inc. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
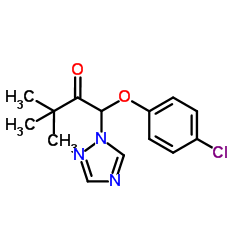 |
Triadimefon
CAS:43121-43-3 |
C14H16ClN3O2 |
|
Is the amphibian X. laevis WEC a good alternative method to ...
2011-09-01 [Reprod. Toxicol. 32(2) , 220-6, (2011)] |
|
Changes of thyroid hormone levels and related gene expressio...
2011-11-01 [Environ. Toxicol. Pharmacol. 32(3) , 472-7, (2011)] |
|
Persistence of repeated triadimefon application and its impa...
2012-01-01 [J. Environ. Sci. Health B 47(2) , 104-10, (2012)] |
|
Low concentrations of atrazine, glyphosate, 2,4-dichlorophen...
2010-01-01 [J. Environ. Sci. (China) 22(9) , 1305-8, (2010)] |
|
Analysis of microRNA and gene expression profiling in triazo...
2013-01-07 [Toxicology 303 , 94-8, (2013)] |