| Structure | Name/CAS No. | Articles |
|---|---|---|
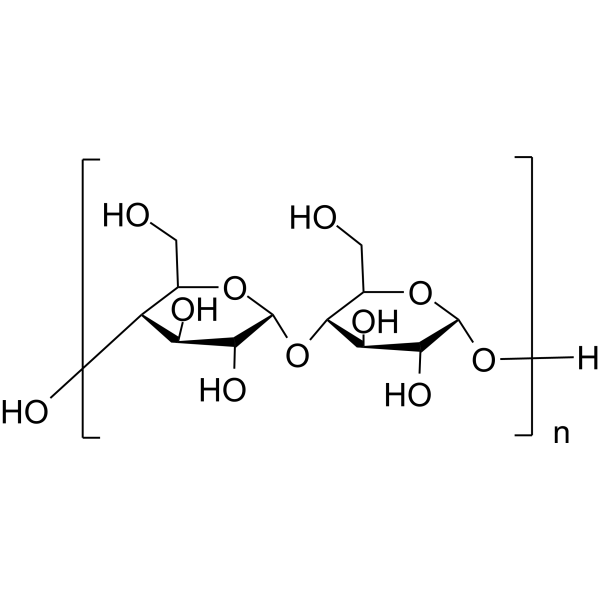 |
Starch
CAS:9005-25-8 |
|
 |
α-Amylase
CAS:9000-90-2 |
|
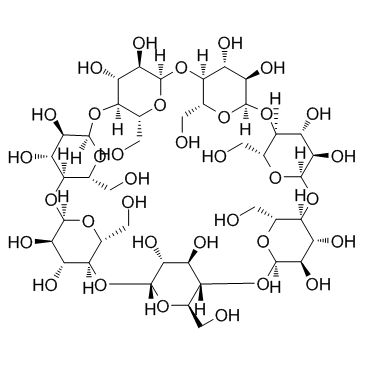 |
β-cyclodextrin
CAS:7585-39-9 |
|
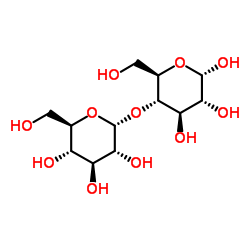 |
Starch soluble
CAS:9005-84-9 |