Aminations of guanosine and deoxyguanosine with hydroxylamine-O-sulfonic acid and 2,4-dinitrophenoxyamine. Dependence on the reaction medium.
K Kohda, K Baba, Y Kawazoe
Index: Nucleic Acids Symp. Ser. (17) , 145-8, (1986)
Full Text: HTML
Abstract
Amination of guanosine (Guo) with 2,4-dinitrophenoxyamine in aqueous DMF gave 7-amino-Guo, which was readily converted to 8,5'-O-cyclo-Guo, and 8-hydroxy-Guo. Deoxyguanosine (dG) gave only deglycosylated 7-amino-G under the same reaction condition. Aminations of Guo and dG with hydroxylamine-O-sulfonic acid above pH 9 gave the corresponding 1-amino derivatives, whereas those in acidic media at pH 2-4 gave 8-amino-Guo and 7-amino-G as the main products, respectively. Amination of Guo in neutral media gave 1-amino-Guo and decomposed products of 7-amino-Guo. The mechanisms of these amination reactions are described.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
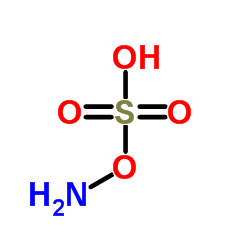 |
Hydroxylamine-O-Sulfonic Acid
CAS:2950-43-8 |
H3NO4S |
|
Elusive metal-free primary amination of arylboronic acids: s...
2012-11-07 [J. Am. Chem. Soc. 134(44) , 18253-6, (2012)] |
|
Co-immobilization of semaphorin3A and nerve growth factor to...
2015-12-01 [Acta Biomater. 28 , 33-44, (2015)] |
|
Mechanism of metal-mediated DNA damage induced by metabolite...
2001-08-08 [Mutat. Res. 479(1-2) , 101-11, (2001)] |
|
The reaction of organoboranes with chloramine and with hydro...
[J. Am. Chem. Soc. 86(17) , 3565-3566, (1964)] |
|
Hydroxylamine-O-sulfonic Acid. Erdik E and Saczewski ...
[e-EROS Encyclopedia of Reagents for Organic Synthesis. , (2013)] |