Elusive metal-free primary amination of arylboronic acids: synthetic studies and mechanism by density functional theory.
Chen Zhu, Gongqiang Li, Daniel H Ess, John R Falck, László Kürti
Index: J. Am. Chem. Soc. 134(44) , 18253-6, (2012)
Full Text: HTML
Abstract
Herein, we disclose the first metal-free synthesis of primary aromatic amines from arylboronic acids, a reaction that has eluded synthetic chemists for decades. This remarkable transformation affords structurally diverse primary arylamines in good chemical yields, including a variety of halogenated primary anilines that often cannot be prepared via transition-metal-catalyzed amination. The reaction is operationally simple, requires only a slight excess of aminating agent, proceeds under neutral or basic conditions, and, importantly, can be scaled up to provide multigram quantities of primary anilines. Density functional calculations reveal that the most likely mechanism involves a facile 1,2-aryl migration and that the presence of an ortho nitro group in the aminating agent plays a critical role in lowering the free energy barrier of the 1,2-aryl migration step.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
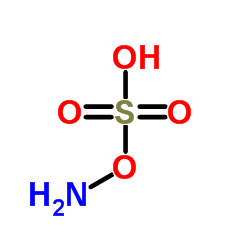 |
Hydroxylamine-O-Sulfonic Acid
CAS:2950-43-8 |
H3NO4S |
|
Co-immobilization of semaphorin3A and nerve growth factor to...
2015-12-01 [Acta Biomater. 28 , 33-44, (2015)] |
|
Aminations of guanosine and deoxyguanosine with hydroxylamin...
1986-01-01 [Nucleic Acids Symp. Ser. (17) , 145-8, (1986)] |
|
Mechanism of metal-mediated DNA damage induced by metabolite...
2001-08-08 [Mutat. Res. 479(1-2) , 101-11, (2001)] |
|
The reaction of organoboranes with chloramine and with hydro...
[J. Am. Chem. Soc. 86(17) , 3565-3566, (1964)] |
|
Hydroxylamine-O-sulfonic Acid. Erdik E and Saczewski ...
[e-EROS Encyclopedia of Reagents for Organic Synthesis. , (2013)] |