| Structure | Name/CAS No. | Articles |
|---|---|---|
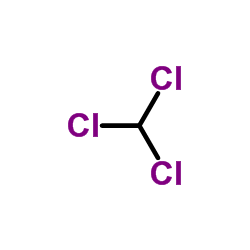 |
Chloroform
CAS:67-66-3 |
|
 |
N-hexane
CAS:110-54-3 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
 |
Antimony trichloride
CAS:10025-91-9 |
|
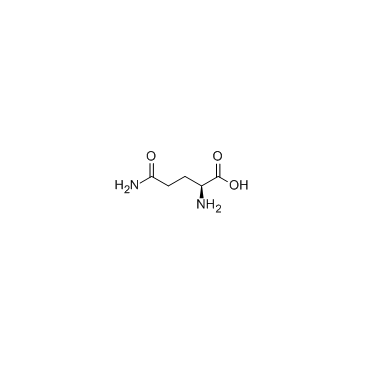 |
L-Glutamine
CAS:56-85-9 |
|
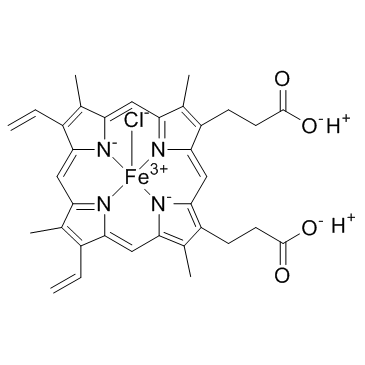 |
Hemin
CAS:16009-13-5 |
|
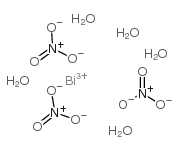 |
Bismuth nitrate pentahydrate
CAS:10035-06-0 |
|
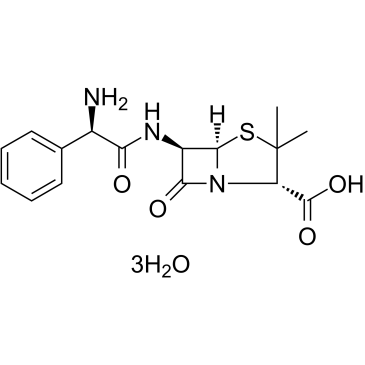 |
Ampicillin Trihydrate
CAS:7177-48-2 |
|
 |
Ampicillin
CAS:69-53-4 |