| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
 |
Acetone
CAS:67-64-1 |
|
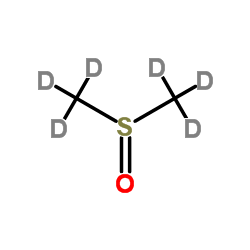 |
DIMETHYL SULFOXIDE-D6
CAS:2206-27-1 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
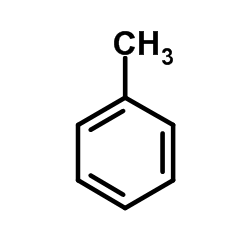 |
Toluene
CAS:108-88-3 |
|
 |
vinyl acetate
CAS:108-05-4 |