| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
 |
L-Nicotine
CAS:54-11-5 |
|
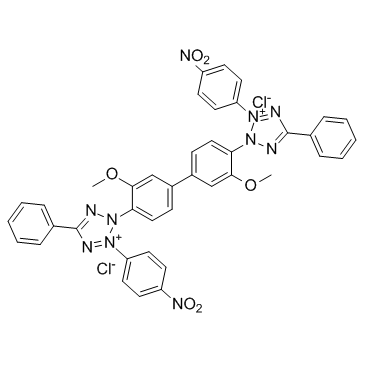 |
NBT
CAS:298-83-9 |
|
 |
Resorufin
CAS:635-78-9 |
|
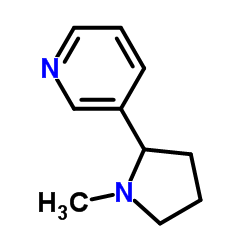 |
(±)-nicotine
CAS:22083-74-5 |
|
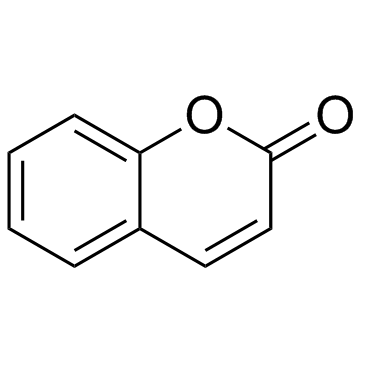 |
Coumarin
CAS:91-64-5 |
|
 |
7-Hydroxycoumarine
CAS:93-35-6 |
|
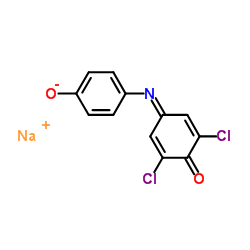 |
Tillman's Reagent
CAS:620-45-1 |
|
 |
7-Methoxy-4-(trifluoromethyl)coumarin
CAS:575-04-2 |