| Structure | Name/CAS No. | Articles |
|---|---|---|
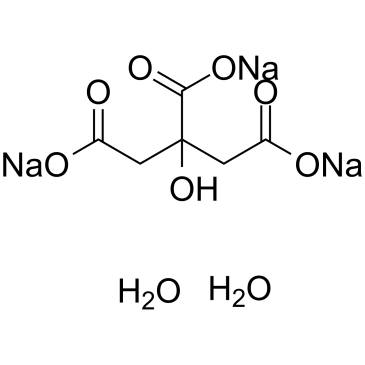 |
Sodium citrate dihydrate
CAS:6132-04-3 |
|
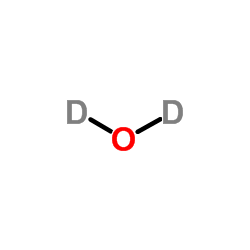 |
Heavy water
CAS:7789-20-0 |
|
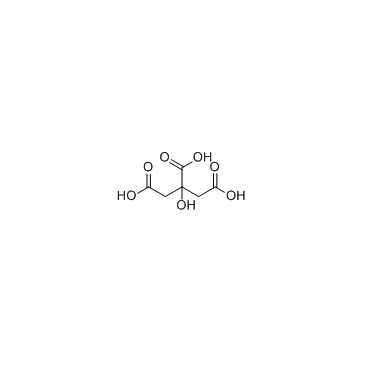 |
Citric Acid
CAS:77-92-9 |
|
 |
Chymotrypsinogen
CAS:9035-75-0 |
|
 |
UNII:TF4710DNP9
CAS:5094-24-6 |