| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Cyclosporin A
CAS:59865-13-3 |
|
 |
Rapamycin (Sirolimus)
CAS:53123-88-9 |
|
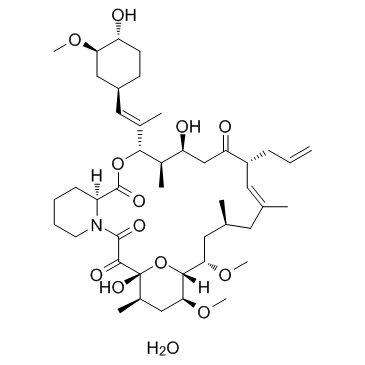 |
Tacrolimus
CAS:109581-93-3 |
|
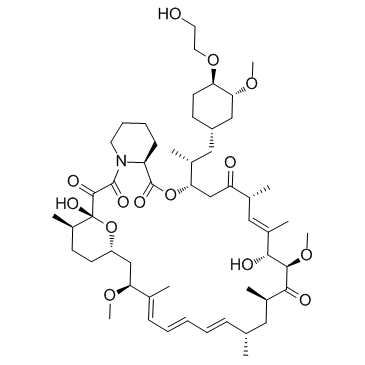 |
Everolimus (RAD001)
CAS:159351-69-6 |