| Structure | Name/CAS No. | Articles |
|---|---|---|
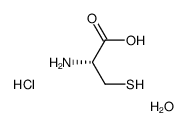 |
l-cysteine hydrochloride hydrate, 98.5
CAS:345909-32-2 |
|
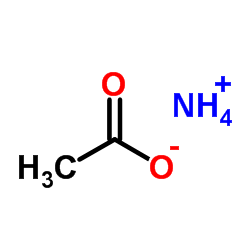 |
Ammonium acetate
CAS:631-61-8 |
|
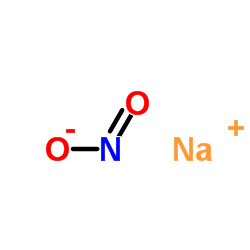 |
Sodium nitrite
CAS:7632-00-0 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Acetylcysteine(N-acetylcysteine)
CAS:616-91-1 |
|
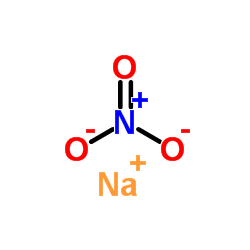 |
sodium nitrate
CAS:7631-99-4 |
|
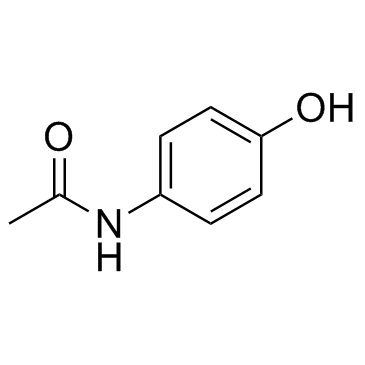 |
4-Acetamidophenol
CAS:103-90-2 |
|
 |
ethyl acetate
CAS:141-78-6 |
|
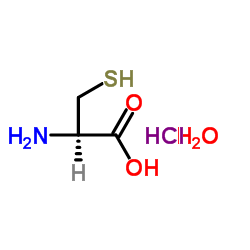 |
L-Cysteine hydrochloride hydrate
CAS:7048-04-6 |
|
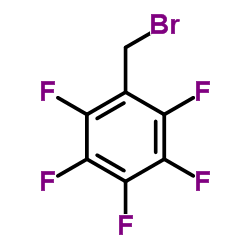 |
Pentafluorobenzyl Bromide
CAS:1765-40-8 |