| Structure | Name/CAS No. | Articles |
|---|---|---|
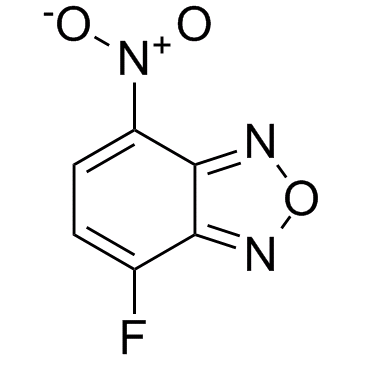 |
4-Fluoro-7-nitrobenzofurazan
CAS:29270-56-2 |
|
 |
Endoproteinase Lys-C
CAS:72561-05-8 |