| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
 |
Chloroform
CAS:67-66-3 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
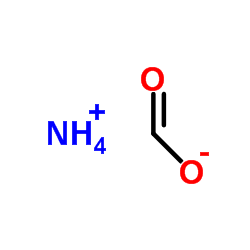 |
Formic acid ammonium salt
CAS:540-69-2 |
|
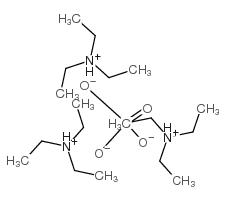 |
Triethylamine Phosphate
CAS:35365-94-7 |