| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
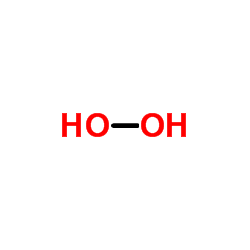 |
Hydrogen peroxide
CAS:7722-84-1 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
L-cysteine
CAS:52-90-4 |
|
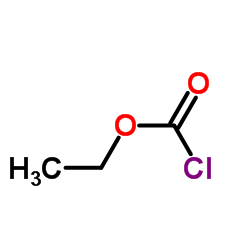 |
Ethyl chloroformate
CAS:541-41-3 |