| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
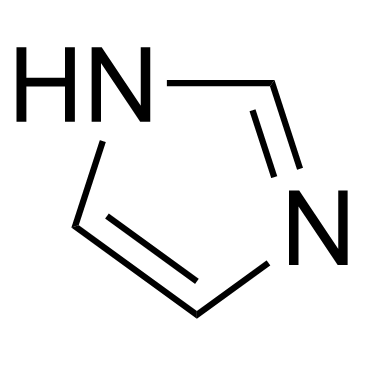 |
Imidazole
CAS:288-32-4 |
|
 |
Ethanol
CAS:64-17-5 |
|
 |
N-hexane
CAS:110-54-3 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
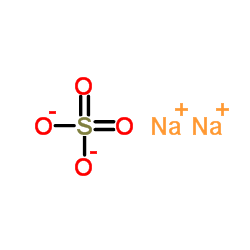 |
sodium sulfate
CAS:7757-82-6 |
|
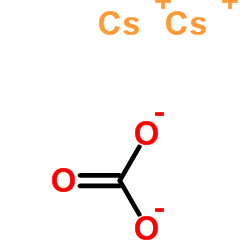 |
Cesium carbonate
CAS:534-17-8 |
|
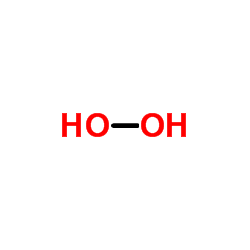 |
Hydrogen peroxide
CAS:7722-84-1 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
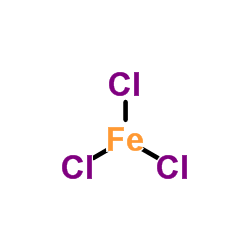 |
Ferric chloride
CAS:7705-08-0 |