| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
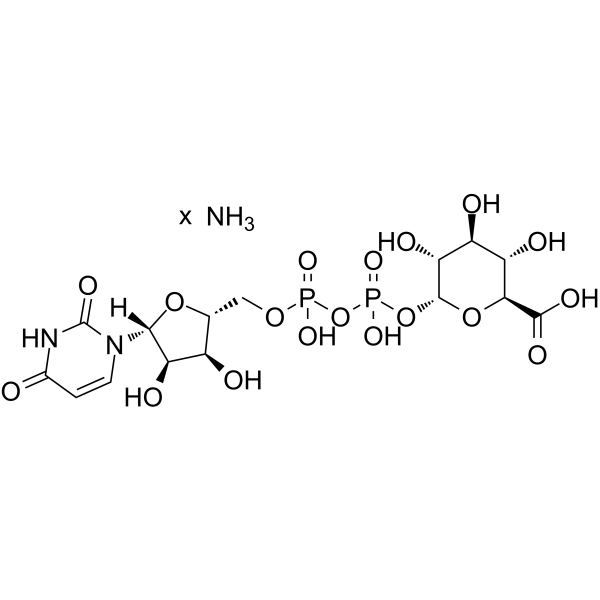 |
Uridine diphosphate glucuronic acid ammonium
CAS:43195-60-4 |
|
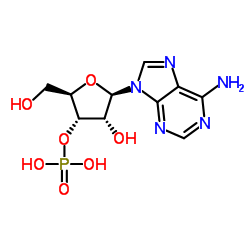 |
3'-Adenylic acid
CAS:84-21-9 |