| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formic Acid
CAS:64-18-6 |
|
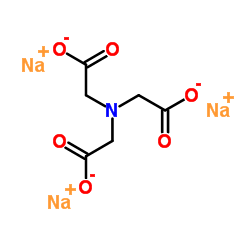 |
SODIUM NITRILOTRIACETATE
CAS:5064-31-3 |
|
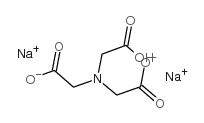 |
Disodium nitrilotriacetate
CAS:15467-20-6 |
|
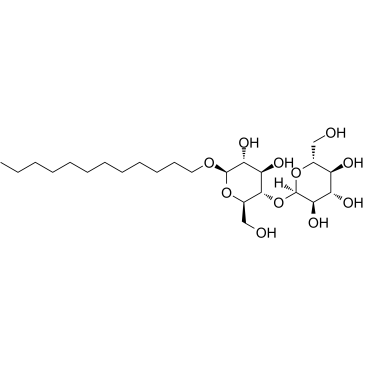 |
n-Dodecyl-beta-D-maltoside
CAS:69227-93-6 |