| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodiumborohydride
CAS:16940-66-2 |
|
 |
Acetone
CAS:67-64-1 |
|
 |
Hydrogen iodide
CAS:10034-85-2 |
|
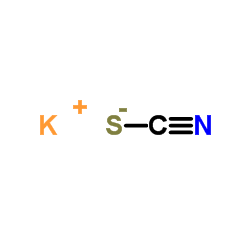 |
Potassium thiocyanate
CAS:333-20-0 |
|
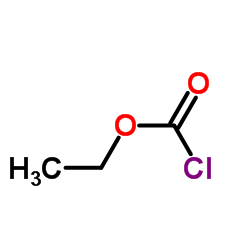 |
Ethyl chloroformate
CAS:541-41-3 |