| Structure | Name/CAS No. | Articles |
|---|---|---|
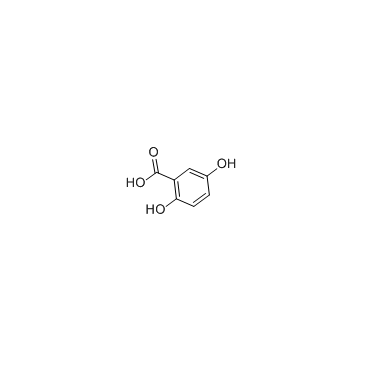 |
Gentisic acid
CAS:490-79-9 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
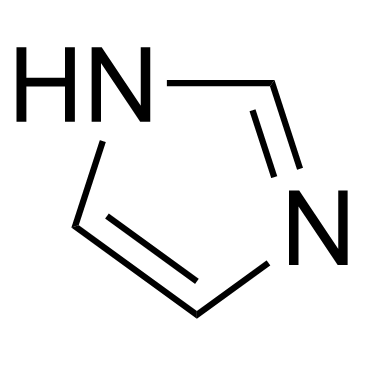 |
Imidazole
CAS:288-32-4 |
|
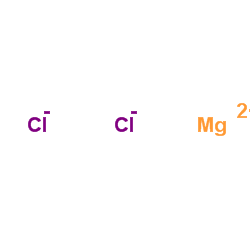 |
Magnesium choride
CAS:7786-30-3 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
 |
H-Gly-Gly-Gly-Gly-OH
CAS:637-84-3 |