| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Oxymetazoline hydrochloride
CAS:2315-02-8 |
|
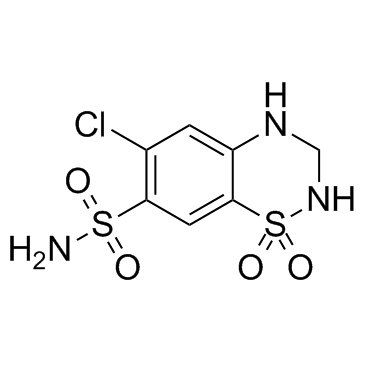 |
Hydrochlorothiazide
CAS:58-93-5 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
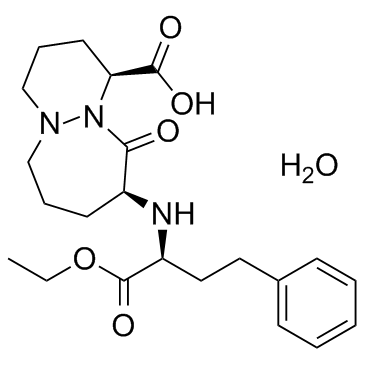 |
Cilazapril Monohydrate
CAS:92077-78-6 |
|
![(1S,9S)-9-{[(2R)-1-Ethoxy-1-oxo-4-phenyl-2-butanyl]amino}-10-oxooctahydro-6H-pyridazino[1,2-a][1,2]diazepine-1-carboxylic acid Structure](https://image.chemsrc.com/caspic/453/106928-09-0.png) |
(1S,9S)-9-{[(2R)-1-Ethoxy-1-oxo-4-phenyl-2-butanyl]amino}-10-oxooctahydro-6H-pyridazino[1,2-a][1,2]diazepine-1-carboxylic acid
CAS:106928-09-0 |