| Structure | Name/CAS No. | Articles |
|---|---|---|
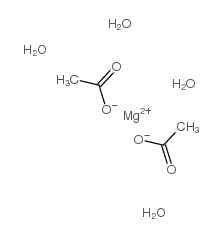 |
Magnesium acetate tetrahydrate
CAS:16674-78-5 |
|
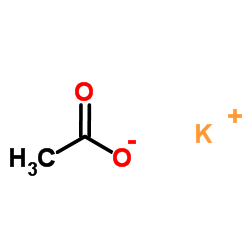 |
Potassium acetate
CAS:127-08-2 |
|
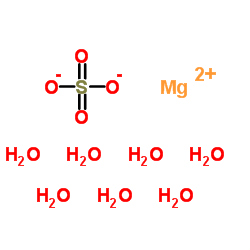 |
magnesium sulfate heptahydrate
CAS:10034-99-8 |
|
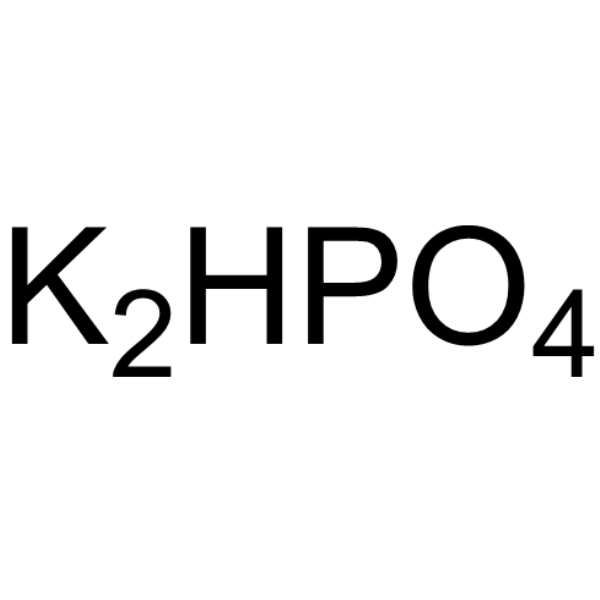 |
Di-potassium monohydrogen phosphate
CAS:7758-11-4 |
|
 |
magnesium sulfate
CAS:7487-88-9 |
|
 |
Water
CAS:7732-18-5 |
|
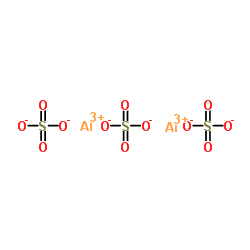 |
Aluminium sulfate
CAS:10043-01-3 |
|
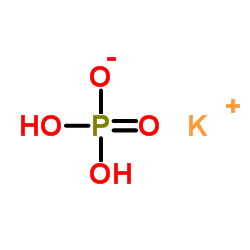 |
Monopotassium phosphate
CAS:7778-77-0 |
|
 |
Potassium Sodium L-(+)-TartrateTetrahydrate
CAS:6381-59-5 |
|
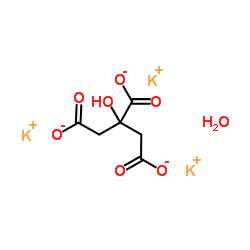 |
Hydroxycitric acid tripotassium hydrate
CAS:6100-05-6 |