| Structure | Name/CAS No. | Articles |
|---|---|---|
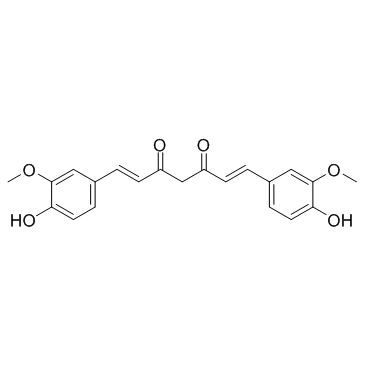 |
Curcumin
CAS:458-37-7 |
|
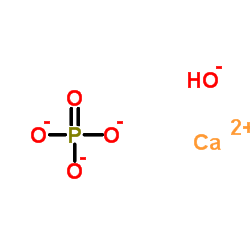 |
Hydroxylapatite
CAS:1306-06-5 |
|
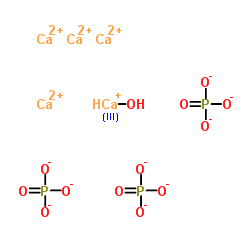 |
Calcium hydroxycalcium(1+) phosphate (4:1:3)
CAS:12167-74-7 |
|
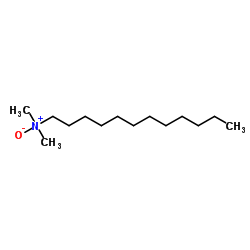 |
N-Dodecyl-N,N-dimethylamine oxide
CAS:1643-20-5 |
|
 |
Galanin (1-13)-Substance P (5-11) amide
CAS:138579-66-5 |