| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium carbonate
CAS:497-19-8 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
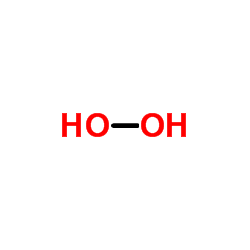 |
Hydrogen peroxide
CAS:7722-84-1 |
|
 |
L-cysteine
CAS:52-90-4 |
|
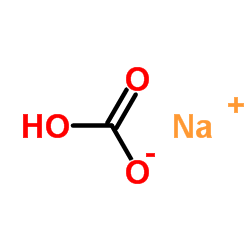 |
SodiuM bicarbonate
CAS:144-55-8 |
|
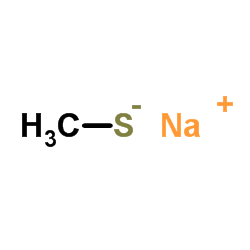 |
Sodium thiomethoxide
CAS:5188-07-8 |
|
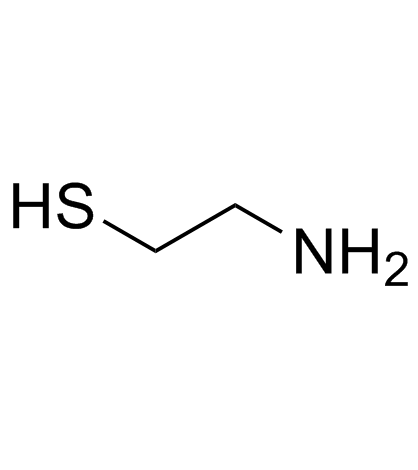 |
2-Aminoethanethiol
CAS:60-23-1 |
|
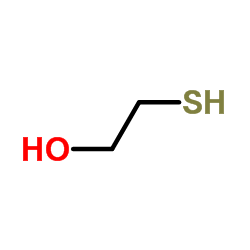 |
mercaptoethanol
CAS:60-24-2 |
|
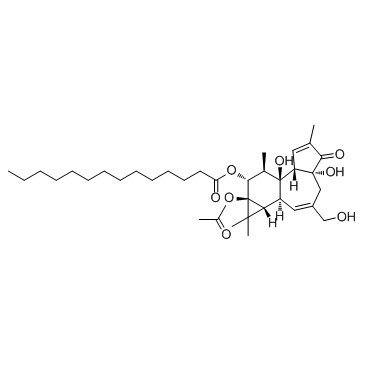 |
12-O-tetradecanoylphorbol-13-acetate
CAS:16561-29-8 |
|
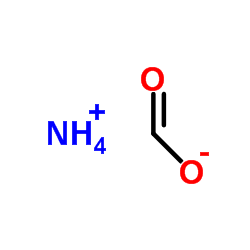 |
Formic acid ammonium salt
CAS:540-69-2 |