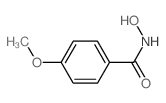
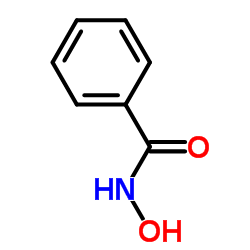
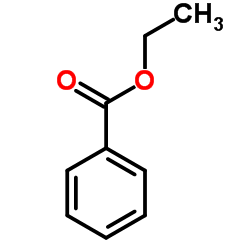
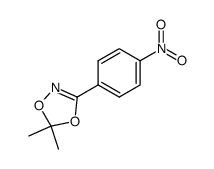
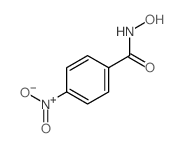
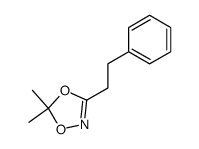
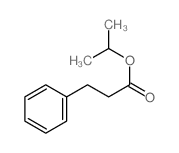
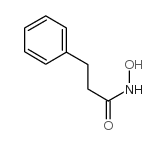
|
~0% |
|
~91% |
|
~85% |
|
~97% |
|
~0% |
|
~0% |