| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethidium bromide
CAS:1239-45-8 |
|
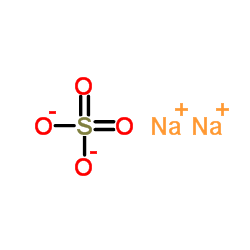 |
sodium sulfate
CAS:7757-82-6 |
|
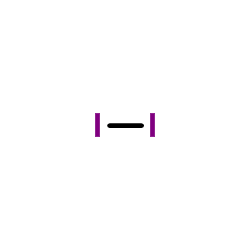 |
molecular iodine
CAS:7553-56-2 |
|
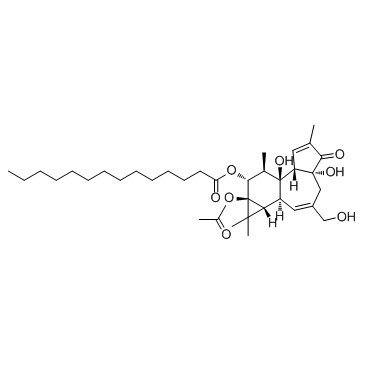 |
12-O-tetradecanoylphorbol-13-acetate
CAS:16561-29-8 |
|
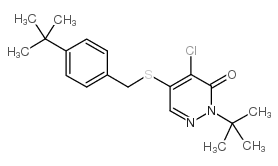 |
Pyridaben
CAS:96489-71-3 |
|
 |
Lithium bis(trimethylsilyl)amide
CAS:4039-32-1 |
|
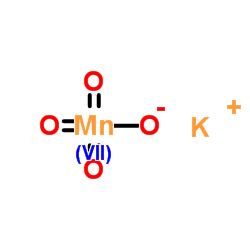 |
Potassium permanganate
CAS:7722-64-7 |
|
 |
argon-40
CAS:1290046-39-7 |