| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethidium bromide
CAS:1239-45-8 |
|
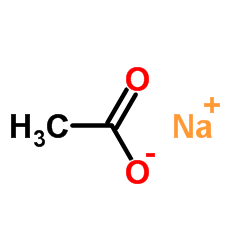 |
Sodium acetate
CAS:127-09-3 |
|
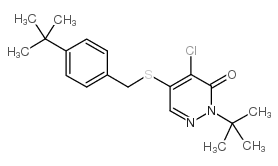 |
Pyridaben
CAS:96489-71-3 |
|
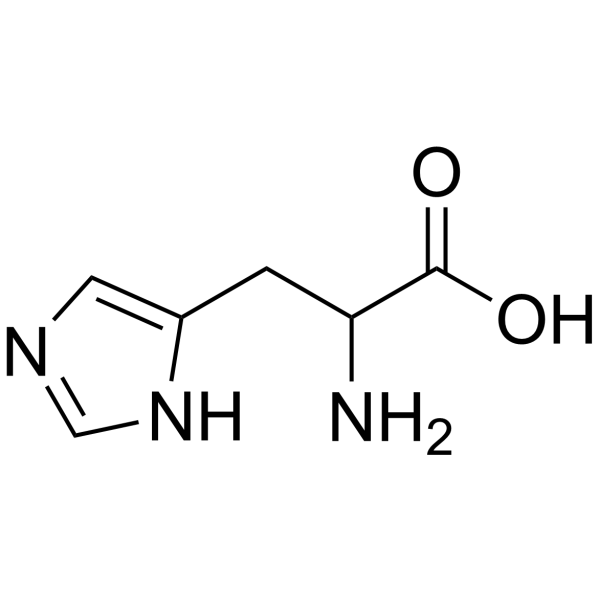 |
DL-Histidine
CAS:4998-57-6 |