| Structure | Name/CAS No. | Articles |
|---|---|---|
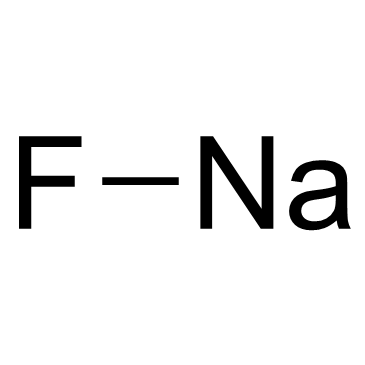 |
Sodium Fluoride
CAS:7681-49-4 |
|
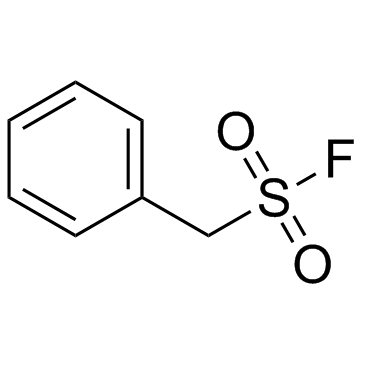 |
PMSF
CAS:329-98-6 |
|
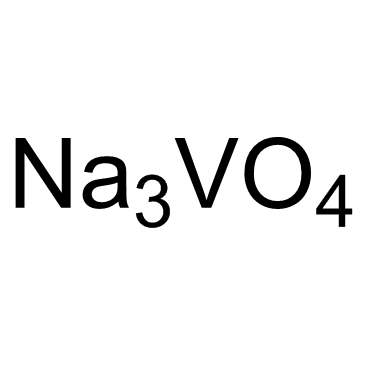 |
Sodium orthovanadate
CAS:13721-39-6 |
|
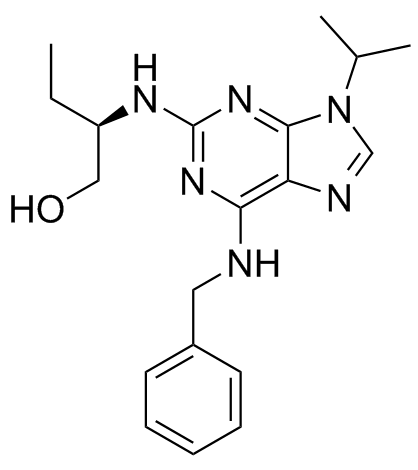 |
Roscovitine (CYC202)
CAS:186692-46-6 |
|
 |
O-Phospho-L-serine
CAS:407-41-0 |