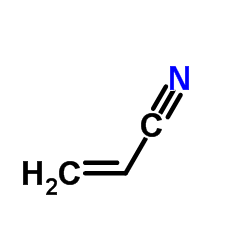
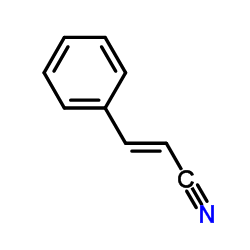
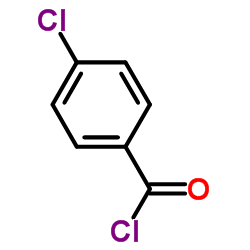
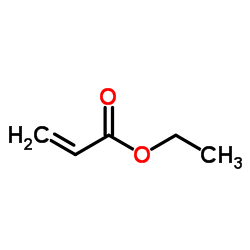
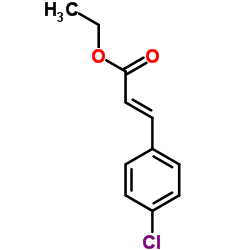
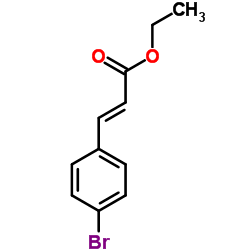
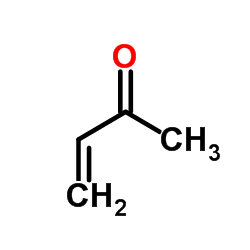
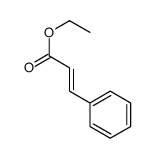
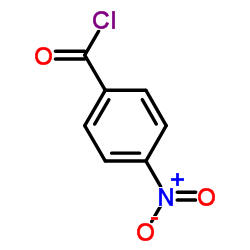
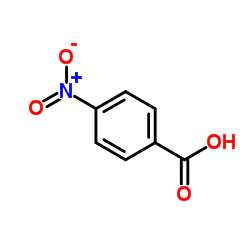
|
~53% |
|
~80% |
|
~76% |
|
~48% |
|
~94% |
|
~56% |
|
~% |