| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
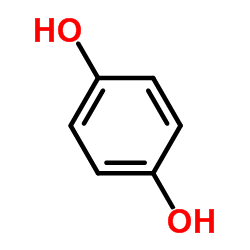 |
Hydroquinone
CAS:123-31-9 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |