| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
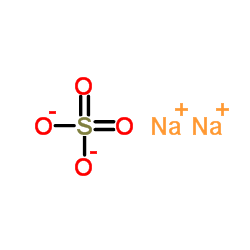 |
sodium sulfate
CAS:7757-82-6 |
|
 |
Methanol
CAS:67-56-1 |
|
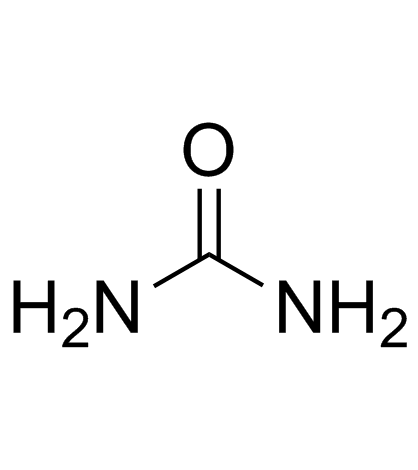 |
Urea
CAS:57-13-6 |
|
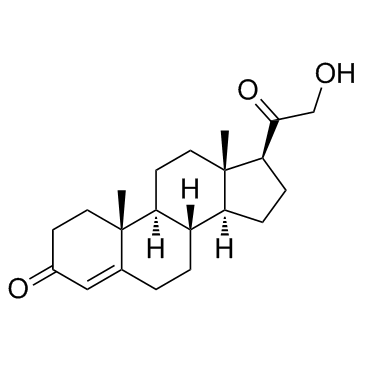 |
Desoxycorticosterone
CAS:64-85-7 |