| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
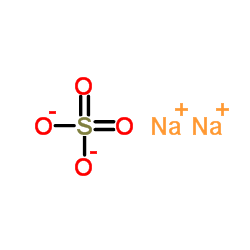 |
sodium sulfate
CAS:7757-82-6 |
|
 |
Potassium iodide
CAS:7681-11-0 |
|
 |
Phenol
CAS:108-95-2 |
|
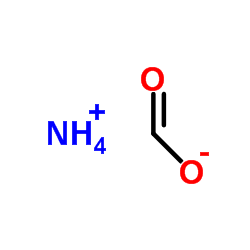 |
Formic acid ammonium salt
CAS:540-69-2 |
|
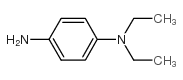 |
N,N-Diethyl-1,4-phenylenediamine
CAS:93-05-0 |
|
 |
Ammonium Chloride
CAS:12125-02-9 |