| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
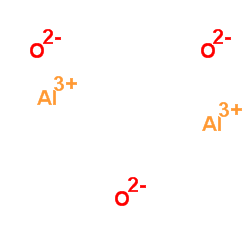 |
Aluminum oxide
CAS:1344-28-1 |
|
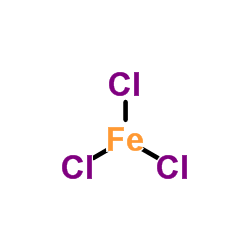 |
Ferric chloride
CAS:7705-08-0 |
|
 |
Ferric chloride hexahydrate
CAS:10025-77-1 |