| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Sulfuric acid
CAS:7664-93-9 |
|
 |
Formaldehyde
CAS:50-00-0 |
|
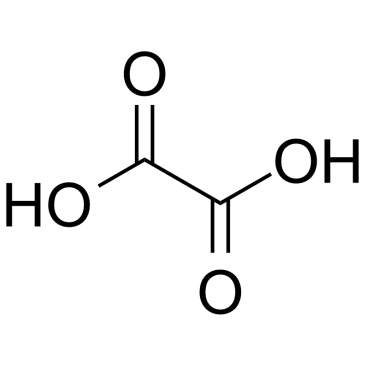 |
Oxalic acid
CAS:144-62-7 |
|
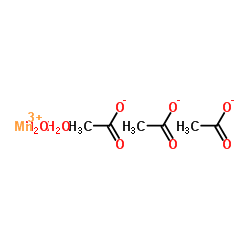 |
Triacetoxymanganese dihydrate
CAS:19513-05-4 |
|
 |
Manganese
CAS:7439-96-5 |
|
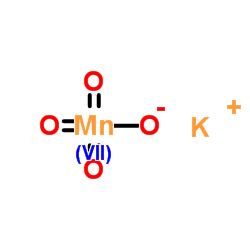 |
Potassium permanganate
CAS:7722-64-7 |
|
 |
Glyoxylic acid
CAS:298-12-4 |
|
 |
Bis(hydroxyammonium) sulfate
CAS:10039-54-0 |