| Structure | Name/CAS No. | Articles |
|---|---|---|
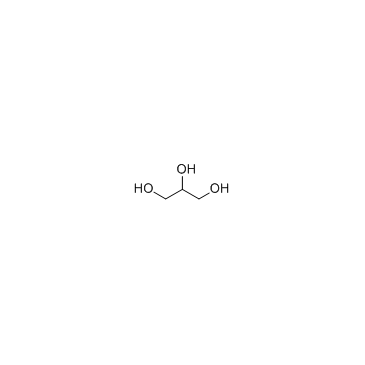 |
Glycerol
CAS:56-81-5 |
|
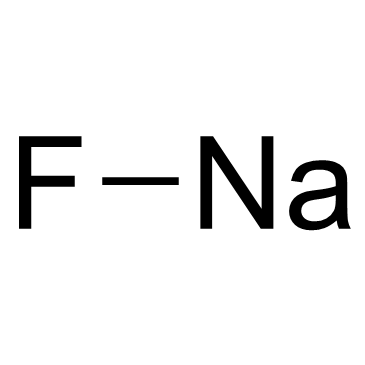 |
Sodium Fluoride
CAS:7681-49-4 |
|
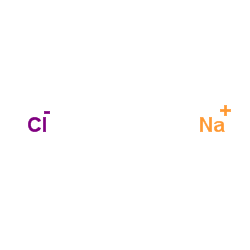 |
sodium chloride
CAS:7647-14-5 |
|
 |
Forskolin
CAS:66575-29-9 |
|
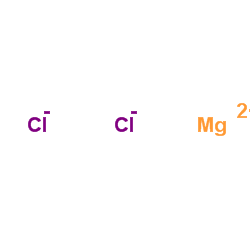 |
Magnesium choride
CAS:7786-30-3 |
|
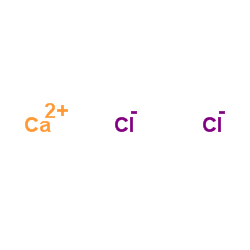 |
Calcium chloride
CAS:10043-52-4 |
|
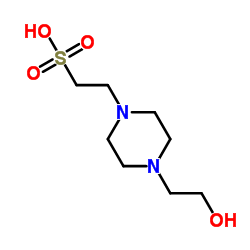 |
HEPES
CAS:7365-45-9 |
|
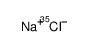 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
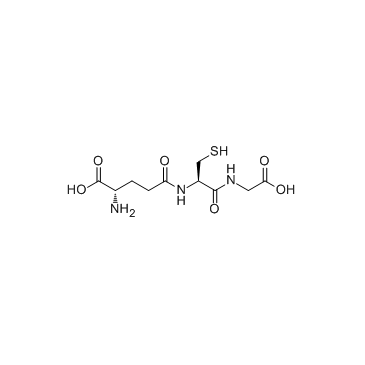 |
Glutathione
CAS:70-18-8 |
|
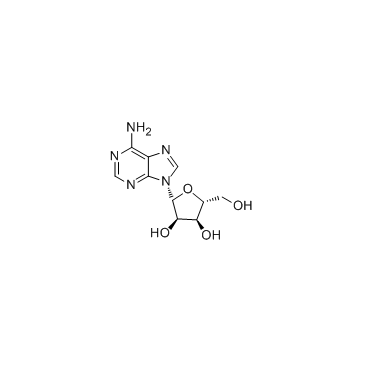 |
Adenosine
CAS:58-61-7 |