|
~% |
|
~% |
|
~% |
|
~% |
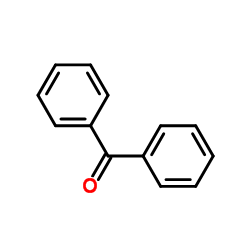
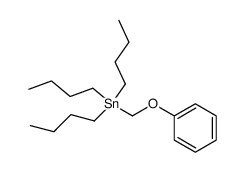
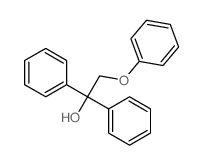
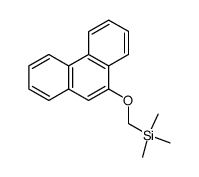
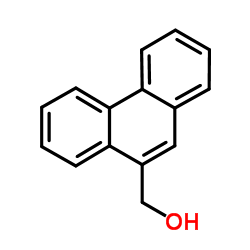
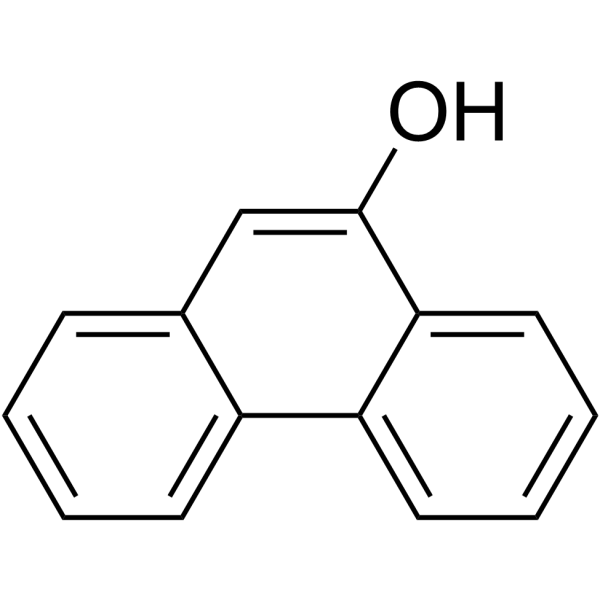
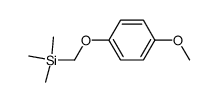
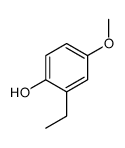
![4-methoxy-2-[2-(trimethylsilyl)ethyl]phenol Structure](https://image.chemsrc.com/caspic/351/83693-59-8.png)
