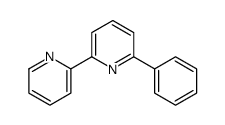
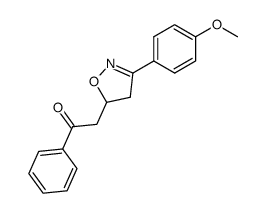
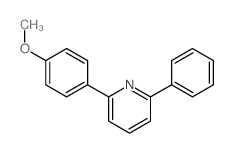
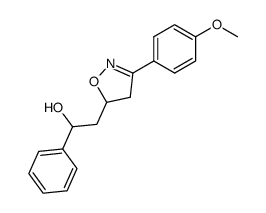
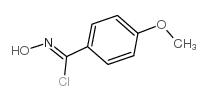
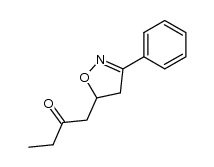
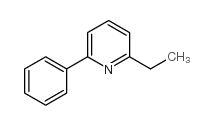
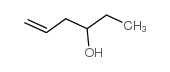
|
~53% |
|
~% |
|
~% |
|
~% |
|
~49% |
|
~% |
|
~% |
|
~39% |
|
~81% |
|
~96% |
|
~% |
|
~% |
|
~% |
|
~12% |
|
~% |
|
~% |
|
~% |
|
~60% |
|
~58% |
|
~% |
|
~% |
|
~% |
|
~50% |
|
~% |
|
~% |
|
~% |

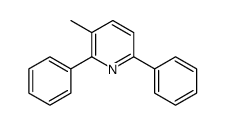

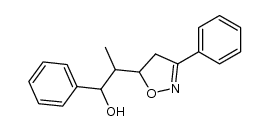
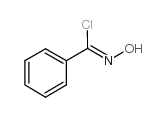
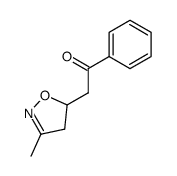
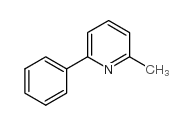
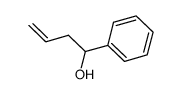
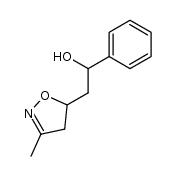
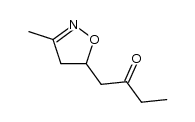
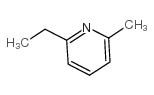
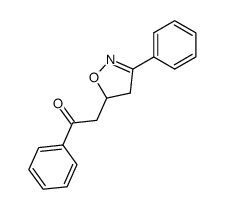
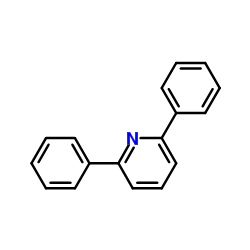
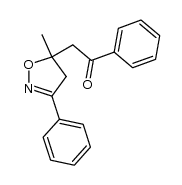
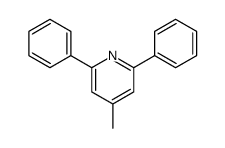
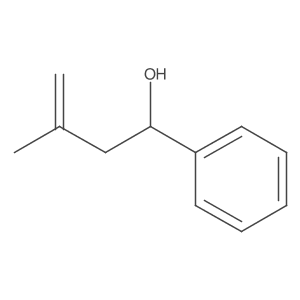

![5-[2-Oxo-2-(2-pyridyl)ethyl]-3-phenyl-2-isoxazoline Structure](https://image.chemsrc.com/caspic/201/134370-32-4.png)