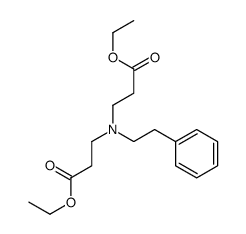
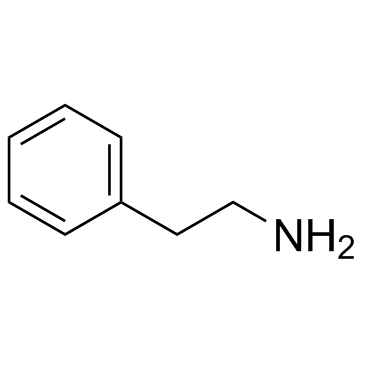
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
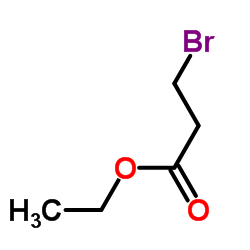
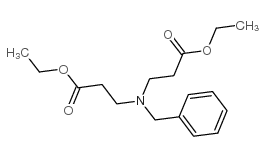
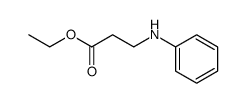
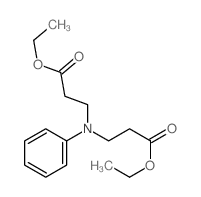

![ethyl [N-(2-phenylethyl)-3-amino]propionate Structure](https://image.chemsrc.com/caspic/309/3936-54-7.png)