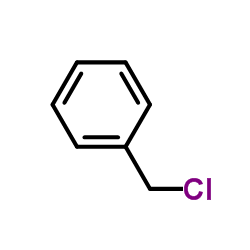
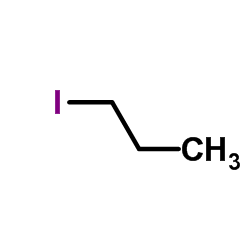
|
~97% |
|
~99% |
|
~92% |
|
~88% |
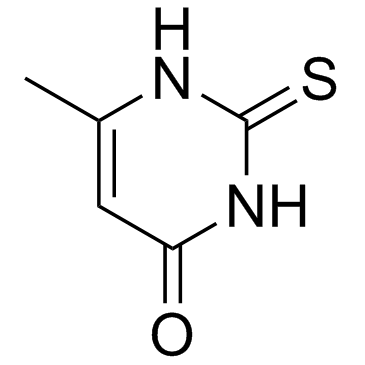
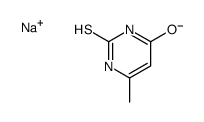

![4(1H)-Pyrimidinone, 6-methyl-2-[(phenylmethyl)thio] Structure](https://image.chemsrc.com/caspic/289/3019-17-8.png)