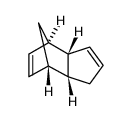
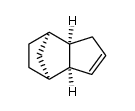
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~0% |
|
~% |
|
~% |
![syn-Tricyclo[4.2.1.12,5]dec-3-en-9-on Structure](https://image.chemsrc.com/caspic/423/67009-89-6.png)
![(1R,2R,6S,7S)-Tricyclo[5.2.1.02,6]decane Structure](https://image.chemsrc.com/caspic/310/2825-83-4.png)
![anti-tricyclo[4.2.1.12,5]dec-2-en-9-one Structure](https://image.chemsrc.com/caspic/211/66953-28-4.png)
![syn-tricyclo[4.2.1.12,5]dec-3-en-9-endo-ol Structure](https://image.chemsrc.com/caspic/176/110848-72-1.png)
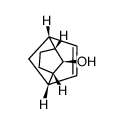
![anti-Tricyclo[4.2.1.12,5]decan-9-on Structure](https://image.chemsrc.com/caspic/068/67009-90-9.png)
![anti-tricyclo[4.2.1.12,5]decan-9-one Structure](https://image.chemsrc.com/caspic/173/66953-29-5.png)
![anti-tricyclo[4.2.1.12,5]decan-3-exo-ol Structure](https://image.chemsrc.com/caspic/197/66337-85-7.png)

![syn-tricyclo[4.2.1.12,5]decan-3-exo-ol Structure](https://image.chemsrc.com/caspic/459/72244-11-2.png)