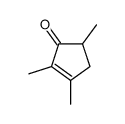
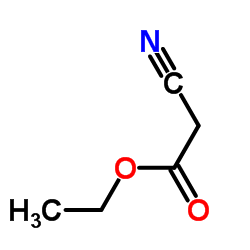
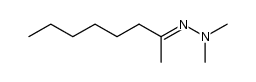
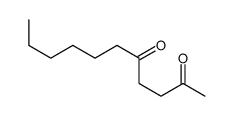
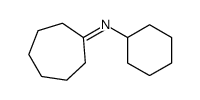
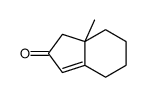
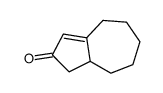
|
~% |
|
~% |
|
~% |
|
~% |
|
~99% |
|
~71% |
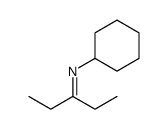
silane Structure](https://image.chemsrc.com/caspic/438/76634-95-2.png)