|
~65% |
|
~% |
|
~% |
|
~% |
|
~76% |
|
~83% |
|
~91% |
|
~60% |
|
~% |
|
~% |
|
~84% |
|
~70% |
|
~% |
|
~% |
|
~60% |
|
~% |
|
~% |
|
~% |
|
~88% |
|
~62% |
|
~% |
|
~% |
|
~% |
|
~70% |
|
~% |
|
~% |
|
~63% |
|
~% |
|
~% |
|
~% |
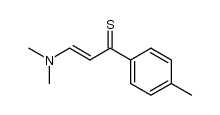
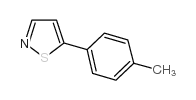

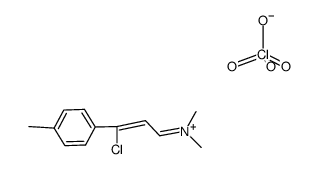
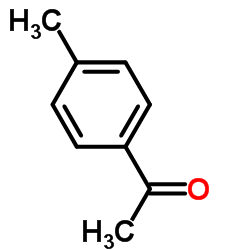
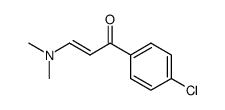
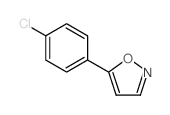

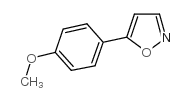
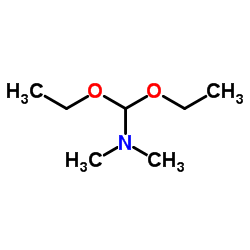
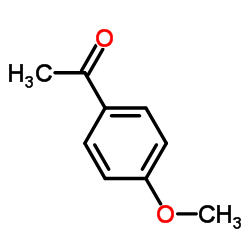
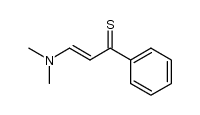
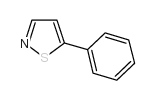
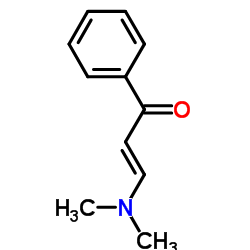


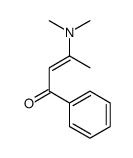
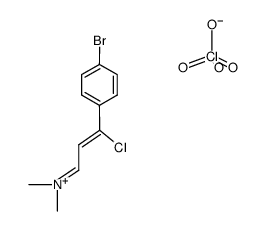

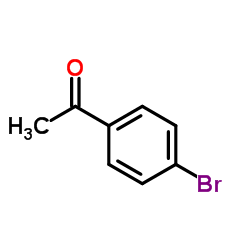
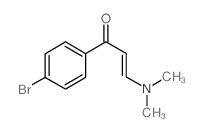
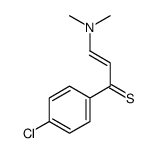
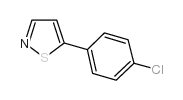
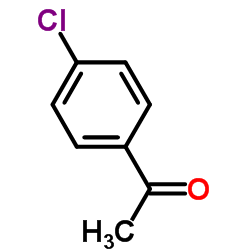
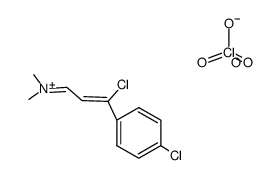
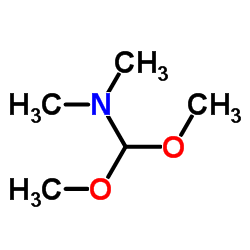
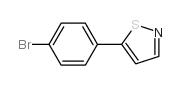
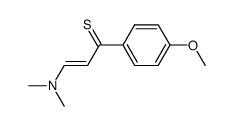
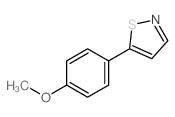
AMMONIUM PERCHLORATE Structure](https://image.chemsrc.com/caspic/184/39812-29-8.png)