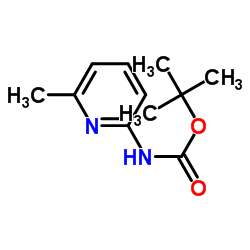
|
~88% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![2-[(3-hydroxy-1-propyl)amino]pyridine-N-oxide Structure](https://image.chemsrc.com/caspic/152/187339-14-6.png)

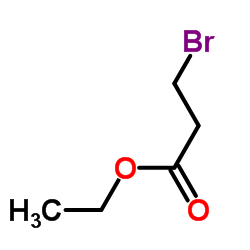
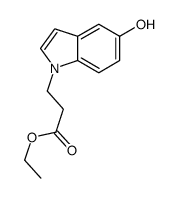
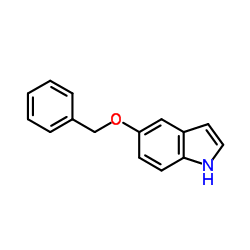

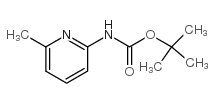
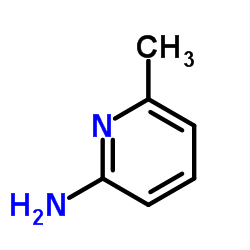
![ETHYL 2-[6-(METHYLAMINO)-2-PYRIDYL]ACETATE Structure](https://image.chemsrc.com/caspic/104/205676-86-4.png)