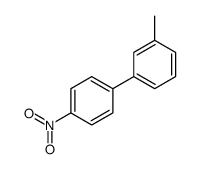
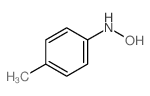
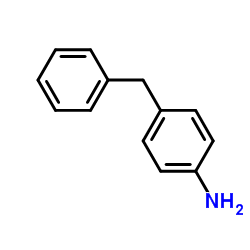
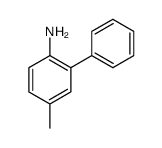
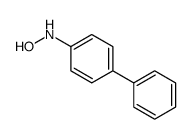
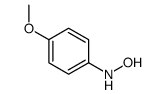
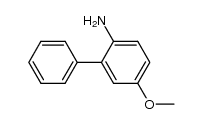
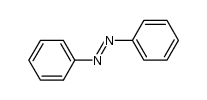
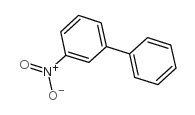
|
~0% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~9%
Detail
|
|
~9%
Detail
|
|
~7% |
|
~72% |
|
~44%
Detail
|
|
~% |
|
~17%
Detail
|
|
~18%
Detail
|
|
~37%
Detail
|
|
~39% |
|
~64% |
|
~64% |
|
~% |
|
~3%
Detail
|
|
~27%
Detail
|
|
~17%
Detail
|
|
~2%
Detail
|
|
~3%
Detail
|
|
~1%
Detail
|
|
~3%
Detail
|
|
~1%
Detail
|
|
~2%
Detail
|
|
~3%
Detail
|
|
~3%
Detail
|
|
~1%
Detail
|
|
~1%
Detail
|
|
~% |
|
~0%
Detail
|
|
~24%
Detail
|
|
~0% |
|
~% |
|
~0%
Detail
|
|
~0%
Detail
|
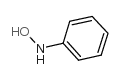
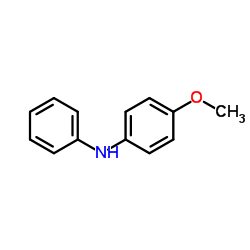
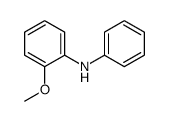
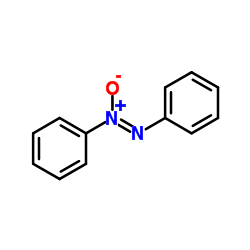
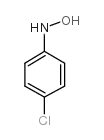
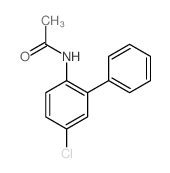

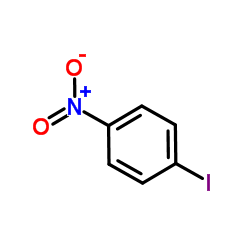
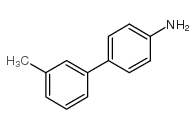
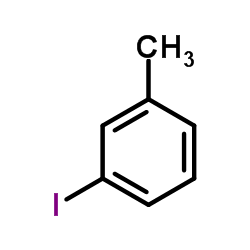
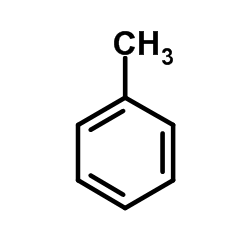

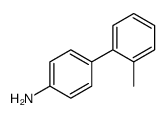
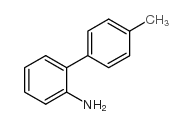
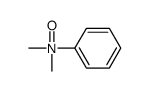
![[1,1'-Biphenyl]-4-amine,N,N-dimethyl Structure](https://image.chemsrc.com/caspic/481/1137-79-7.png)
![N,N-dimethyl-[1,1'-biphenyl]-2-amine Structure](https://image.chemsrc.com/caspic/339/6590-81-4.png)
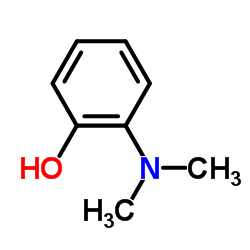
![[1,1':2',1''-Terphenyl]-4'-amine Structure](https://image.chemsrc.com/caspic/370/10569-67-2.png)
![[1,1':3',1''-Terphenyl]-4'-amine Structure](https://image.chemsrc.com/caspic/443/63344-48-9.png)