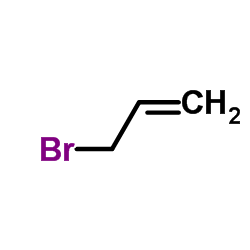
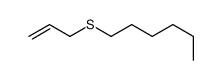
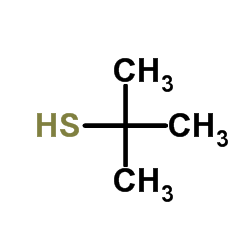
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
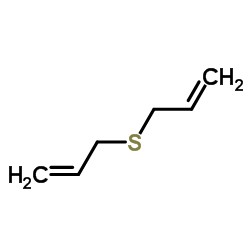

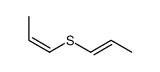
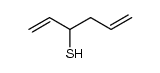
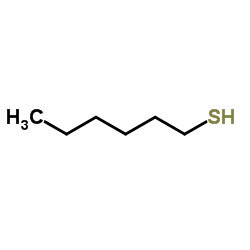
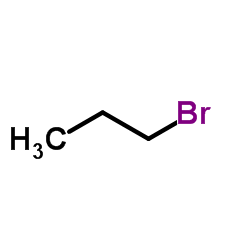

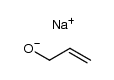

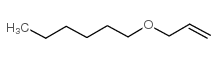

![2-methyl-2-[(Z)-prop-1-enyl]sulfanyl-propane Structure](https://image.chemsrc.com/caspic/027/89795-31-3.png)