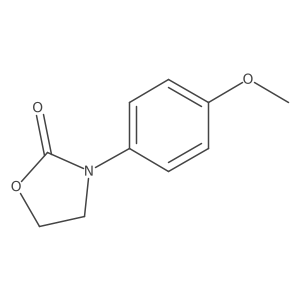
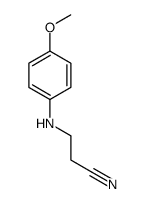
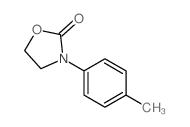
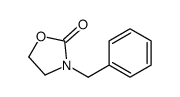
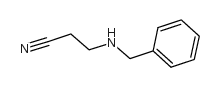
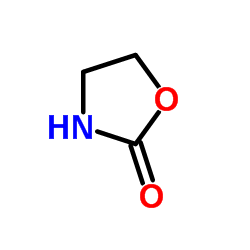
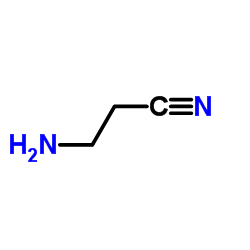
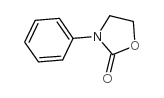
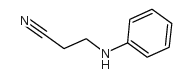
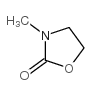
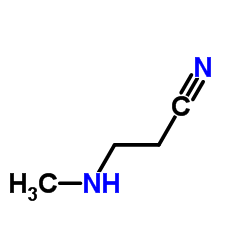
|
~79% |
|
~74% |
|
~67% |
|
~73% |
|
~13% |
|
~82% |
|
~49% |