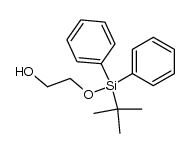
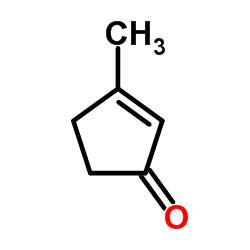
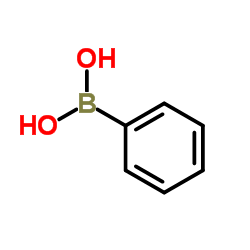
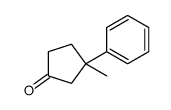
|
~% |
|
~% |
|
~% |
|
~88% |
|
~% |
|
~95% |
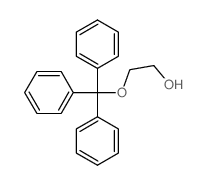
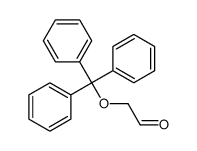
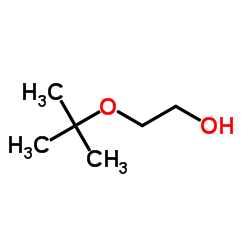
![1-[(2-methylpropan-2-yl)oxy]propan-2-one Structure](https://image.chemsrc.com/caspic/397/28047-99-6.png)
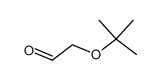
![(2S)-1-{[tert-butyl(diphenyl)silyl]oxy}propan-2-ol Structure](https://image.chemsrc.com/caspic/240/125803-68-1.png)
![1-[tert-butyl(diphenyl)silyl]oxypropan-2-one Structure](https://image.chemsrc.com/caspic/352/118171-02-1.png)