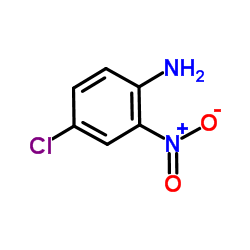
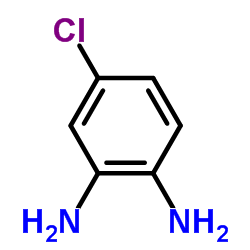
|
~76% |
|
~% |
|
~89% |
|
~% |
|
~% |
|
~34% |
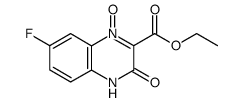
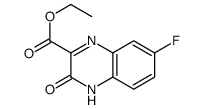

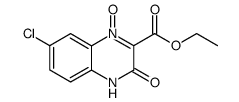
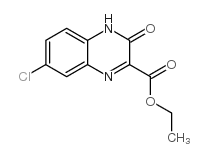
![ethyl 3-[(4-chloro-2-nitrophenyl)amino]-3-oxopropionate Structure](https://image.chemsrc.com/caspic/386/143948-68-9.png)