Intramolecular reactions of allyloxy radicals featuring six-...
[Begley; Housden; Johns; Murphy Tetrahedron, 1991 , vol. 47, # 39 p. 8417 - 8430]
|
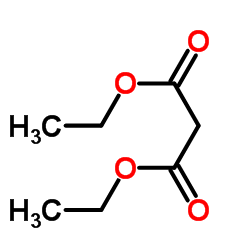
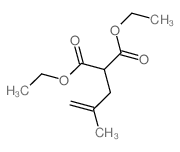
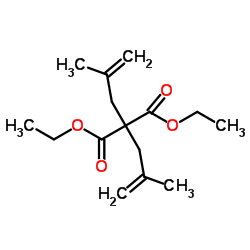
Catalytic deallylation of allyl-and diallylmalonates
[Necas, David; Tursky, Matyas; Kotora, Martin Journal of the American Chemical Society, 2004 , vol. 126, # 33 p. 10222 - 10223]
|
Trimethylenemethane Diyl Mediated Tandem Cycloaddition React...
[Lee, Hee-Yoon; Jung, Yongsik; Yoon, Yeokwon; Kim, Byung Gyu; Kim, Yeonjoon Organic Letters, 2010 , vol. 12, # 11 p. 2672 - 2674]
|
π-Allyl-palladium-komplexe stabilisiert durch den tripodalen...
[Domhoever, Bernd; Klaeui, Wolfgang Journal of Organometallic Chemistry, 1996 , vol. 522, # 2 p. 207 - 212]
|
Catalytic deallylation of allyl-and diallylmalonates
[Necas, David; Tursky, Matyas; Kotora, Martin Journal of the American Chemical Society, 2004 , vol. 126, # 33 p. 10222 - 10223]
|

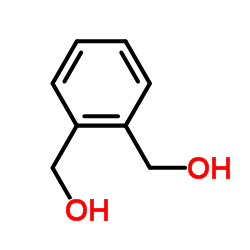
![[2-(prop-2-enoxymethyl)phenyl]methanol Structure](https://image.chemsrc.com/caspic/034/195323-89-8.png)