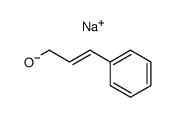
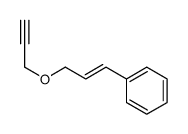
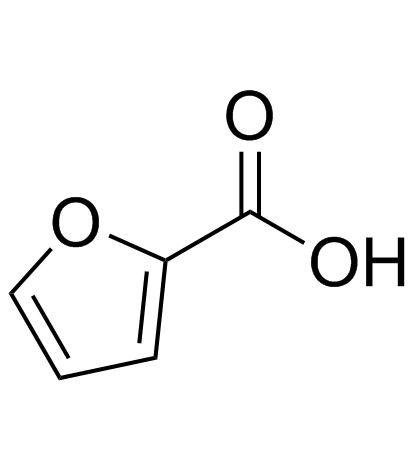
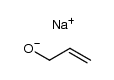
|
~% |
|
~% |
|
~% |
|
~% |
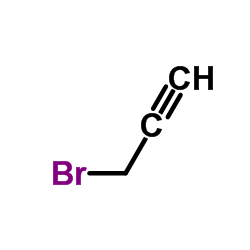

![[(2-Propyn-1-yloxy)methyl]benzene Structure](https://image.chemsrc.com/caspic/413/4039-82-1.png)