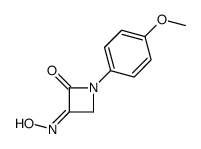
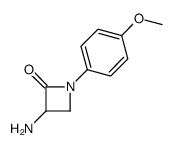
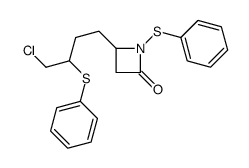
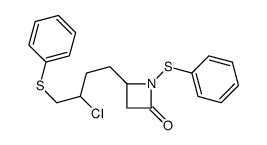
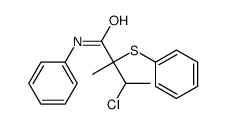
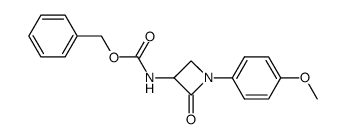
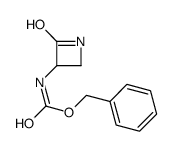
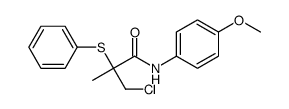
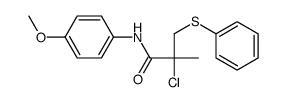
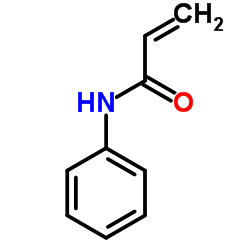
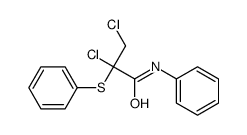
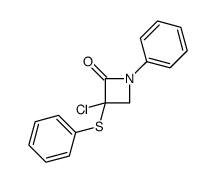
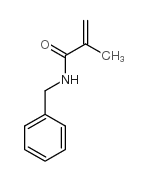
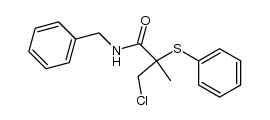
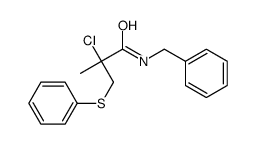
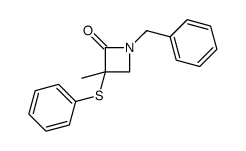
|
~% |
|
~% |
|
~% |
|
~% |
|
~46% |
|
~% |
|
~% |
|
~% |
|
~61% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~75% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~95% |
|
~% |
|
~% |
|
~% |
|
~59% |
|
~79% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~35% |
|
~% |
|
~% |
|
~22% |
|
~% |
|
~94% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~% |
|
~% |
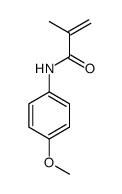
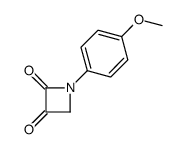
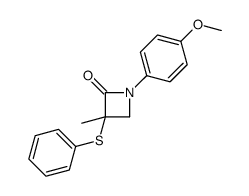
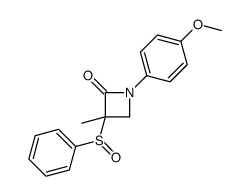
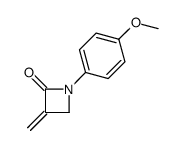

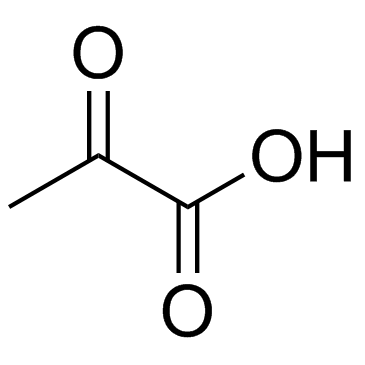
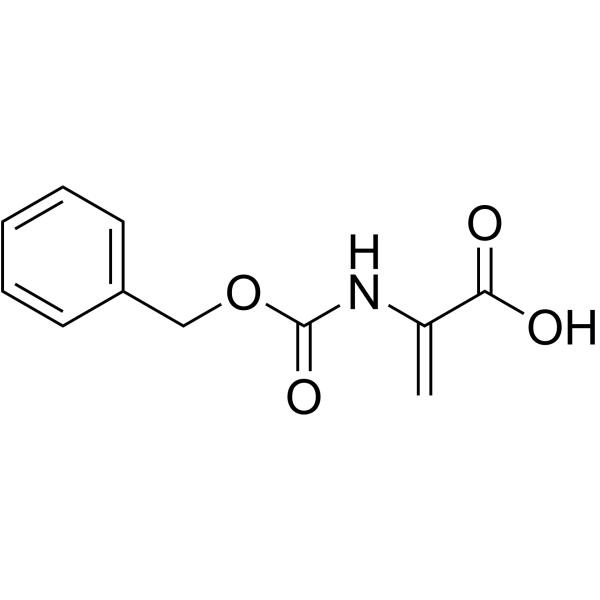
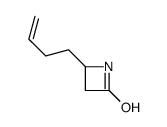
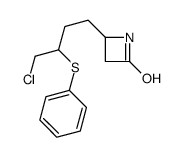
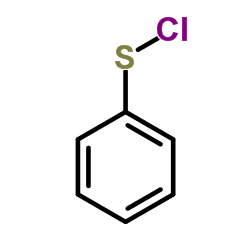
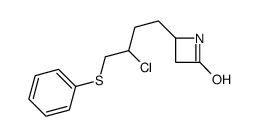
![4-but-3-enyl-1-[tert-butyl(dimethyl)silyl]azetidin-2-one Structure](https://image.chemsrc.com/caspic/170/109975-76-0.png)