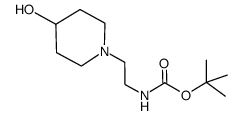
|
~% |
|
~% |
|
~% |
|
~% |
|
~35% |
|
~% |
|
~% |
|
~% |
|
~% |
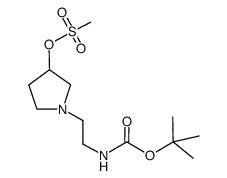
![tert-butyl N-[2-(3-chloropyrrolidin-1-yl)ethyl]carbamate Structure](https://image.chemsrc.com/caspic/026/857637-44-6.png)
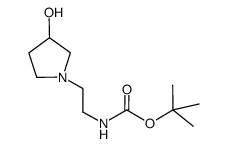


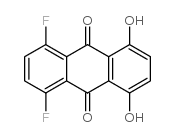
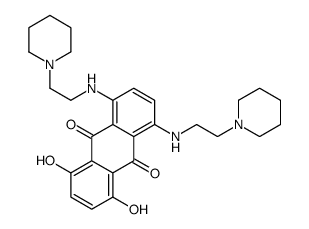

![tert-butyl N-[2-(4-chloropiperidin-1-yl)ethyl]carbamate Structure](https://image.chemsrc.com/caspic/385/857637-30-0.png)